Enthalpy Change of Combustion
Enthalpy is used to calculate minimum power for a compressor. In this equation dW is equal to dW pdV and is known as the boundary work.

Total 2 Average 5 X2f 5 What Is The Heat Of Combustion What Is The Definition Of Enthalpy Chemistry Lessons Chemistry Classroom How To Study Physics
Of molecular Chlorine 0.

. First Law of Thermodynamics The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes. Use this equation to calculate the molar enthalpy change for a reaction. This is the same as the thermodynamic heat of combustion since the enthalpy change for the reaction assumes a common temperature of the compounds before and after combustion in which case the water produced by combustion is condensed to a liquid.
Where m is the mass of the substance that has a temperature change ΔT and a specific heat capacity c. The enthalpy change involved in creating those molecules is the heat of formation of the molecules. Periodic table and energy.
Combustion happens as the gasoline most commonly a fossil fuel reacts with the oxygen in the air to create heat. Enthalpy Formula is denoted as. According to the definition of enthalpy of neutralization chem libretexts the standard enthalpy change of neutralization is the enthalpy change when solutions of an acid and an alkali react together under standard conditions to produce 1 mole of water.
CH 4g 2O 2g CO 2g 2H 2 O l ΔH c -210 kcal. Of molecular Oxygen 0. The heat of formation of molecular hydrogen gas.
The first law of thermodynamics in terms of enthalpy show us why engineers use the enthalpy in thermodynamic cycles eg. It is denoted by ΔH c. The higher heating value takes.
The first law makes use of the key concepts of internal energy heat and system workIt is used extensively in the discussion of heat enginesThe standard unit for all these quantities would be the joule. Enthalpy Change Heat of the Reaction. The standard enthalpy change of atomisation ΔH at ꝋ is the enthalpy change when 1 mole of gaseous atoms is formed from its element under standard conditions.
Constant-volume and constant. Using 3 the total sensible and latent heat removed from the air can be calculated as. Calculate the enthalpy change for the combustion of acetylene ceC2H2 Solution.
Because the enthalpy of all the starting materials is zero. Brayton cycle or Rankine cycle. The heat change q in a reaction is given by the equation q mcΔT.
So just a quick tabulation then. Enthalpy change occurs during a change in the state of matter. DU dQ dW.
I highlighted 1 mole of water because thats what I used to solve the problem. The enthalpy change that takes place when one mole of compound is completely burnt in excess of air or oxygen. Remember the combustion of a hydrocarbon requires oxygen and results in the production of carbon dioxide and water.
According to the law of energy conservation the change in internal energy is equal to the heat transferred to less the work done by the system. As for example the heat of combustion of methane is given below. If the only work done is a change of volume at constant pressure the enthalpy change is exactly equal to the heat transferred to the system.
Students should be able to. There are two kinds of enthalpy of combustion called higher and lower heating value. Change in enthalpy is used to measure heat flow in calorimetry.
The heat produced by the combustion of fossil fuels is used in the operation of machineries such as boilers furnaces ovens and engines. As the enthalpy change amplifies itself as heat the statement heat of reaction is frequently made use of in place of enthalpy change of the reaction. The enthalpy change that takes place when one gram.
The combustion of acetylene. Standard conditions in this syllabus are a temperature of. Boundary work occurs because the mass of the substance.
The classical form of the law is the following equation. When energy needs to be added to a material to change its phase from a liquid to a gas that. There are many other applications of enthalpy in thermal engineering.
Of molecular Bromine 30907 kilojoules per. Unless the pressure is extremely high the work done by applied pressure on solids and liquids can be neglected and enthalpy can be represented by the internal energy component alone. H t 1202 kgm 3 1 m 3 s 77 kJkg dry air - 28 kJkg dry air 589 kW Imperial Units.
Heat of combustion. From the Mollier diagram we estimate the water enthalpy in the hot air to be 77 kJkg dry air and the enthalpy in the cold air to be 28 kJkg dry air. It is measured to evaluate a throttling process or Joule-Thomson expansion.
The enthalpy of products is H2 and is less than the heat content of reactants H1. In chemistry and thermodynamics the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is recommended by IUPAC. Enthalpy change of atomisation.
The change in internal energy with respect to change in temperature at fixed volume is the Specific Heat at constant volume - c v. The combustion reaction happens as the fuel and oxygen react creating fire or heat and light. 1 The first step is to make sure that the equation is balanced and correct.
Enthalpy change ΔH refers to the amount of heat energy transferred during a chemical reaction at a constant pressure.
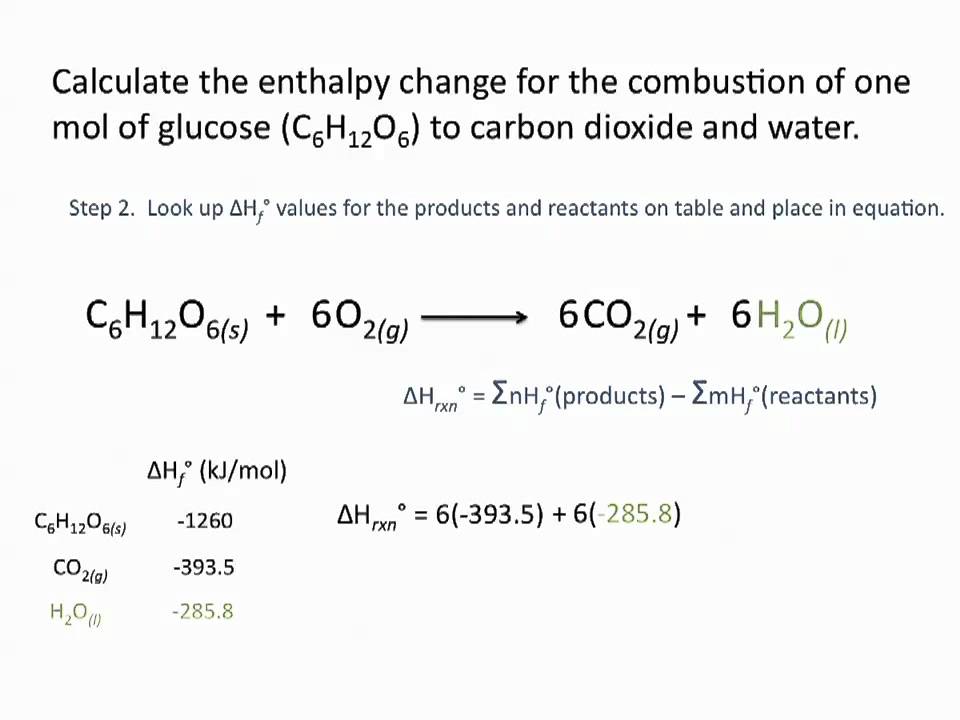
Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial

Total 2 Average 5 X2f 5 What Is The Heat Of Combustion What Is The Definition Of Enthalpy Of Comb Heat Energy Chemical Reactions Exothermic Reaction

Enthalpy Heat Combustion Experiment 4 Energy Level Heat Exothermic Reaction

No comments for "Enthalpy Change of Combustion"
Post a Comment